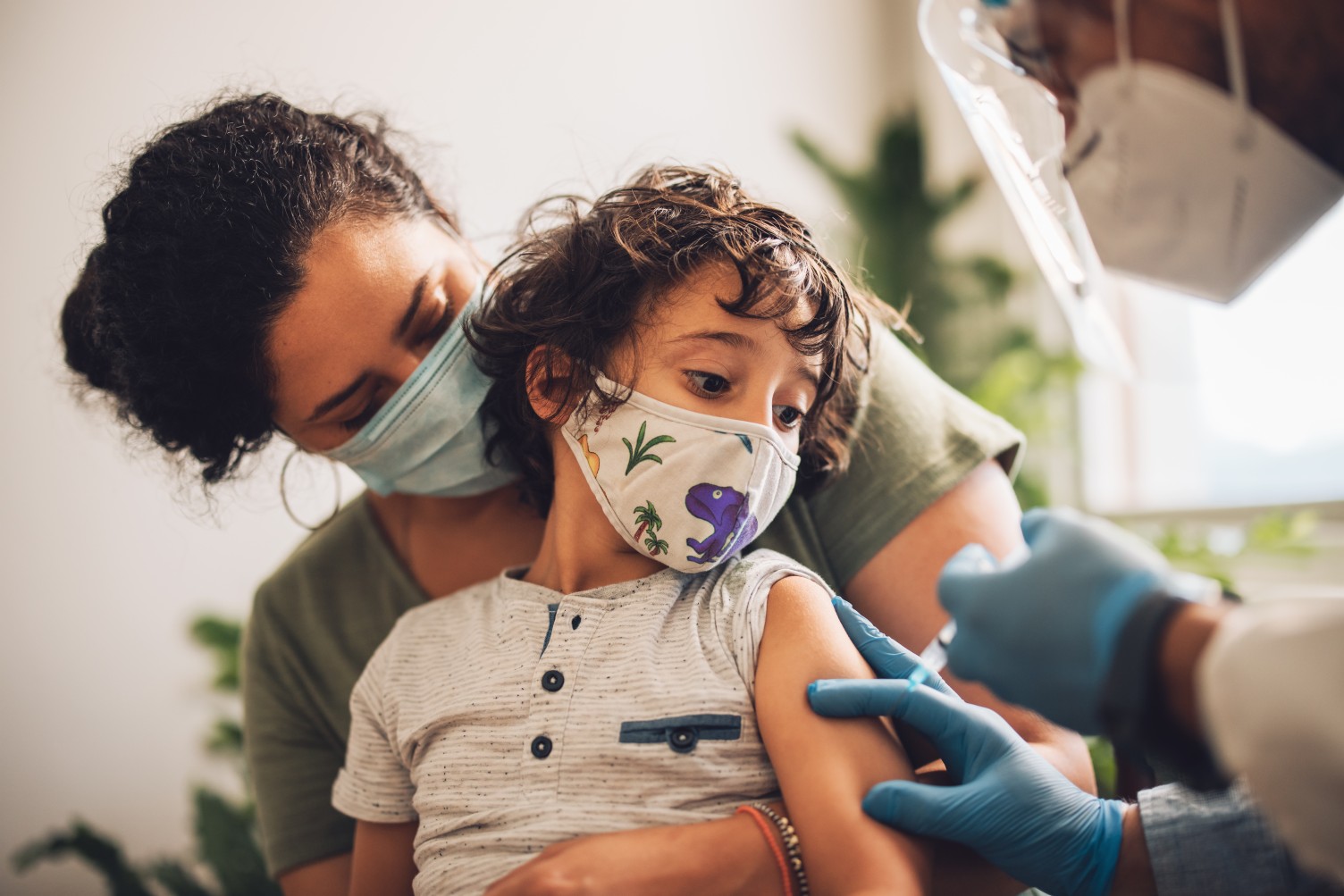
As parents across the nation await the news of vaccine availability for their children, the White House is ready to meet this demand. Here is what you need to know to bring you up to date on the ongoing COVID-19 crisis and the efforts to vaccinate more Americans.
The administration of President Joe Biden announced that it is ready to roll out the vaccine to children ages 5 to 11, assuming US Food and Drug Administration (FDA) approval. The White House said that it has enough vaccines for all 28 million American children in this age group. The plan will provide over 25,000 pediatric and other primary care medical offices with the vaccine it needs to meet the needs of their community. In addition, the Biden administration will ask various rural health clinics and pharmacies to assist with the mass vaccination once it becomes available.
The plan was laid out on Wednesday by White House COVID-19 response director Jeff Zients. He said that the administration recognizes that children have different needs than adults when it comes to vaccinations. The goal is to offer these shots at places kids feel comfortable and familiar with, such as with their regular healthcare provider instead of mass vaccination sites.
The Department of Health and Human Services (HHS) is also planning on launching a new national public education campaign aimed at parents and guardians. The goal of the program is to provide accurate information about the vaccine along with the risks of COVID-19 for children.
The FDA vaccine advisory group is scheduled to meet on October 26 to consider the Pfizer vaccination for children ages 5 to 11. Should this group give the vaccine the green light, it will move on to the US Centers for Disease Control and Prevention (CDC) for final approval. The vaccine is currently approved by the FDA for people ages 16 and over with an emergency use authorization (EUA) in use for kids ages 12 to 15.
Should the vaccine be provided with the EUA for kids ages 5 to 11, it will be administered as a 10-microgram dose. This is far less than the 30-microgram dose being used for those ages 12 and older.
FDA Authorizes Booster Doses for Moderna and Johnson & Johnson
Also on Wednesday, the FDA authorized booster doses for the Moderna and Johnson & Johnson versions of the vaccine. The FDA also approved the use of any of these three vaccines to be mixed and matched. As a result of the authorization, the Moderna booster is approved for those who were fully vaccinated at least six months and who are either 65 and over or 18 and over with a high risk of developing severe COVID-19. In addition, the booster is authorized for those with occupational exposure to the virus, such as health care workers.
The FDA also authorized a single booster dose of the Johnson & Johnson vaccine for those who received the first shot at least two months ago.
The CDC’s Advisory Committee on Immunization Practices will meet on Wednesday to decide on recommending this official FDA authorization. Assuming the CDC is in agreement with the FDA’s recommendation, it will be up to the CDC director to give the final approval.
New Data Reveals Unvaccinated Risk of COVID-19
A new data tool revealed by the CDC on Wednesday demonstrates that those not vaccinated against COVID-19 are up to 18 times more likely to be hospitalized with virus complications than those who have been vaccinated. CDC Director Dr. Rochelle Walensky released information and graphs demonstrating these statistics.
In addition to the hospitalization data, the new data revealed that unvaccinated individuals had a 6.1 times higher risk of testing positive for the virus. The unvaccinated are also 11.3 times more likely to die as a result of complications from COVID-19.
New CDC COVID-19 Forecasts
The CDC also published new forecasts on Wednesday, showing a little hope on the horizon. According to the latest ensemble forecasts from multiple sources, COVID-19 deaths and hospitalizations are predicted to continue their decrease over the next four weeks. The latest data forecasts a total of 748,000 to 769,000 COVID-19 deaths by November 13.
This projection is down from the last forecast on October 13. As of October 20, there have been 728,313 reported COVID-19 deaths in the US.
New Delta Variant?
Health experts are closely monitoring an emerging subtype of the Delta variant that is raising red flags in the UK. As a descendent of the original Delta variant, the AY.4.2 variant is now responsible for about 6% of new cases in this corner of the world. While some people believe it may be more contagious than the first Delta variant, this has not been proven. At this point, the Delta subtype has only been confirmed in a few cases in Denmark and the US.
New York City Vaccine Mandate Update
The controversial New York City vaccine mandate is now applicable to all municipal employees. Workers who get their first shot between now and October 29 at sites operated by the city will receive a $500 bonus. According to New York City Mayor Bill de Blasio, there are still 46,000 city employees that are not yet vaccinated as of October 20.
Prior to the Wednesday announcement, city employees were required to either be vaccinated or submit to weekly testing. The new mandate takes away testing as an option for compliance.
The city has already mandated that all public school teachers and other educational workers be fully vaccinated. Additionally, all New York City health care workers were mandated to get vaccinated under state law.
The unions representing the city’s police and firefighters have already said that they will push back against the mandate.