The U.S. Food and Drug Administration (FDA) authorized a new COVID-19 vaccine just as an offshoot of the original Omicron strain is spreading like wildfire throughout the country. Here is the latest in the fight against COVID-19.
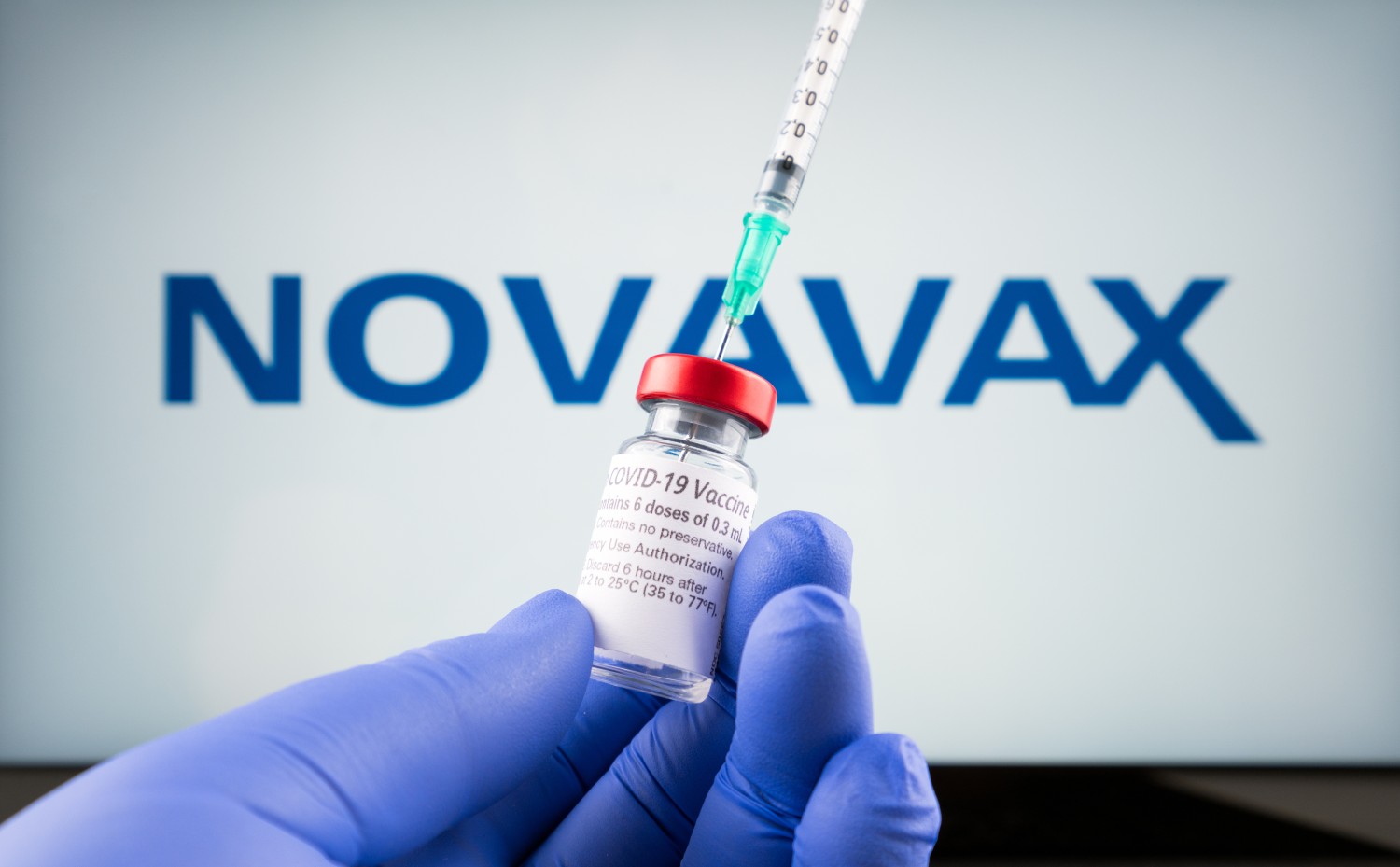
There is a new tool in the arsenal of ways to battle COVID-19. On Wednesday, the FDA issued an emergency use authorization (EUA) for the Novavax COVID-19 vaccine. The protein-based vaccine goes by the name of Adjuvanted. The vaccine is now approved for Americans ages 18 and older.
The Novavax vaccine offers an alternative to the widely used mRNA-based vaccines by Pfizer and Moderna. Novavax applied for the EUA last February, hoping to offer another, more traditional vaccine in addition to the current single-dose Johnson & Johnson non-mRNA option. Because the Johnson & Johnson vaccine has been connected to an increased risk of blood clots, its use has been more limited in the U.S. Experts are hoping that the authorization of another non-mRNA vaccine with Novavax may encourage more Americans to get the shot.
According to Novavax, data from two different studies with over 30,000 participants demonstrate an efficacy of about 90% in preventing significant infection from the virus. The Novavax vaccine is administered in two different doses spaced three weeks apart.
On Monday, the administration of President Joe Biden announced that they have already secured 3.2 million doses of the vaccine. Like the other approved vaccines, this one will also be available for free.
COVID-19 Cases Spiking in U.S.
As has been the case with the previous two summers, COVID-19 is once again spiking in the U.S., particularly in the southern tier of the country. This time it is another variant of the Omicron strain that is wreaking havoc. The BA.5 strain contains three mutations in the spike protein that make it more effective at infecting the cells and escaping the protection offered by vaccines and prior infections.
According to the latest data from the U.S. Centers for Disease Control and Prevention (CDC), the BA.5 strain was responsible for about 2 out of every 3 new cases in the U.S. last week. The strain has outpaced its predecessors for the last two months. What is most alarming about this particular strain is that even those who have had COVID-19 as recently as this winter or spring are becoming infected again.
Current case counts as reported to the CDC are landing at about 110,000 per day. However, experts caution that this number is vastly undercounted. Because most people do not report the results of at-home testing, the actual number is estimated to be about seven times this amount. This also does not include the many asymptomatic cases that go unnoticed.
While it is easy to become complacent with the virus, health officials point out that there are still about 300 to 350 deaths per day at the hands of the virus. In addition, the number of hospitalized patients needing intensive care is up about 23% over the last two weeks.
Another New Variant on the Horizon
As the U.S. battles with the BA.5 variant, there is another mutation that is ripping through other parts of the world. The mutation known as BA.2.75 has now been identified in approximately 10 countries, including a small amount in the U.S. The strain appears to be spreading the most in India. Some experts are predicting that this will be the variant that drives new infections in the U.S. this fall.
Younger Americans Getting Impatient for Boosters
While the virus continues to spread throughout the U.S., medical experts are encouraging those that are eligible for a booster to get one right away. However, a second booster is only authorized for Americans ages 50 and older and those who have underlying health conditions. This is leaving those in the younger age groups frustrated that they cannot yet get the coveted second booster.
The CDC and FDA continue to maintain that they are looking at new data to determine when they will authorize the widespread use of a second booster. All groups above the age of five are now eligible for at least one booster shot.
According to the latest Census data, there are about 139 million American adults between the age of 18 and 50. While many of these people rushed to get the first booster shot last fall, they have not been eligible to receive the second booster, leaving a large part of the population potentially vulnerable to the impacts of the virus.
Did you find this content useful? Feel free to bookmark or to post to your timeline for reference later!